Oxygen FAQ
Up to date, expert answers to frequently asked questions (FAQ) about oxygen supply systems, respiratory care and pulse oximetry written by OCC & collaborators.
Top 10 most popular FAQs
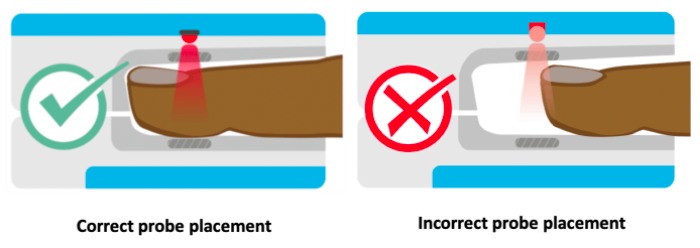
The locations for placing pulse oximeters vary by the type of device and probes available, including whether it is a transmittance or a reflectance device. Transmittance devices shine light through a part of the body, so they must be placed on a relatively thin or translucent area such as a fingertip, earlobe, nose or the foot of an infant. It is important that the body part is placed far enough into the probe so that the light shines through the tissue rather than to the side of it. Reflectance devices do not require light to pass through tissue, so they can be placed in a variety of locations including the patient’s forehead, wrist, foot, or chest.
References: Lifebox Pulse Oximetry Learning Module
Keywords: body part, location, placement, finger
- Always see manufacturer’s specifications.
- All external filters should be inspected at least daily
- For turbine and compressor ventilators, external inlet filters and fan filters must be cleaned (if cleanable per manufacturer) or replaced at least monthly. For ventilators that allow, bacterial/viral filters should be placed proximal to external inlet filters.
- For example, the LTV-1200: The external inlet filter should be removed and cleaned once a month and can be reused. If operated in high dust or humidity environments, it may need to be cleaned more often. The filter can be cleaned with mild detergent and warm water by using a soft cleaning brush. The filter must be rinsed thoroughly of all detergent residue and must be dried completely prior to re-insertion. If the filter is damaged or cannot be thoroughly cleaned, it should be replaced. The external inlet filter appears to be a proprietary item (Reticulated Foam P/N 10258)
- The fan filter should be removed and cleaned at least once a month (same cleaning procedure as described for the inlet filter). It also can be reused. If the ventilator is being operated in high dust or humidity environments, it may need to be cleaned more often. If the filter is damaged or cannot be thoroughly cleaned, it should be replaced.
- The LTV-1200 model also has an oxygen inlet filter that must be inspected and cleaned on a regular basis. It also is cleaned using a mild cleanser, warm water and a soft brush. Rinse the filter thoroughly to remove all traces of the cleanser. Allow the filter to dry completely before replacing it in the ventilator. Inspect the filter for damage. If it is not intact, or shows signs of damage or cannot be completely cleaned, it should be replaced. The filter is a proprietary item (O2 Inlet Filter (P/N 19845-001) and the accompanying O-Ring (P/N10609)
- The Zoll 731 ventilator: has an internal 2-stage filtration system (an outer foam filter and inner disk filter) to protect the gas flow. External filters should be visually inspected on a daily basis (or more frequently) for dust build up during extended operation in harsh environments and changed when they appear dirty. Use of external filters will preserve the life of the proprietary internal filters (foam filter REF#: 465-0028-00, Air Intake Disk Filter (REF # 465-0027-00). If external filters are not (or cannot be) used, the internal filters must be visually inspected on a regular basis and replaced when dirty. Note: proprietary internal filters cannot be cleaned and reused: they must be replaced.
- The Medtronic PB560 has an external air inlet filter that is intended to be replaced (~monthly or more often) rather than reused
Elimination of a machine mounted inspiratory filter – If a bacterial viral filter is used between the circuit Wye and the patient, then an additional inspiratory filter between the machine and the inspiratory limb may not be necessary to protect the patient (so long as the machine is kept clean and an airway mounted filter and/or expiratory limb filter is used). That is a big “if,” and the use of an inspiratory limb filter at the circuit takeoff is to eliminate this chance of error.
A single inspiratory filter setup (at the wye) has been suggested as an option in settings of severe shortage. This setup may create potential for error and subsequent contamination of the machine. Additionally, the use of one instead of two filters in series significantly decreased viral filtration efficiency. The impact on risk of viral contamination is unknown.
Expiratory limb filter extended use (i.e. not changing between patients) has been suggested by some as an option if severe shortage is faced and appropriate bacterial viral filter is used at the patient. APSF
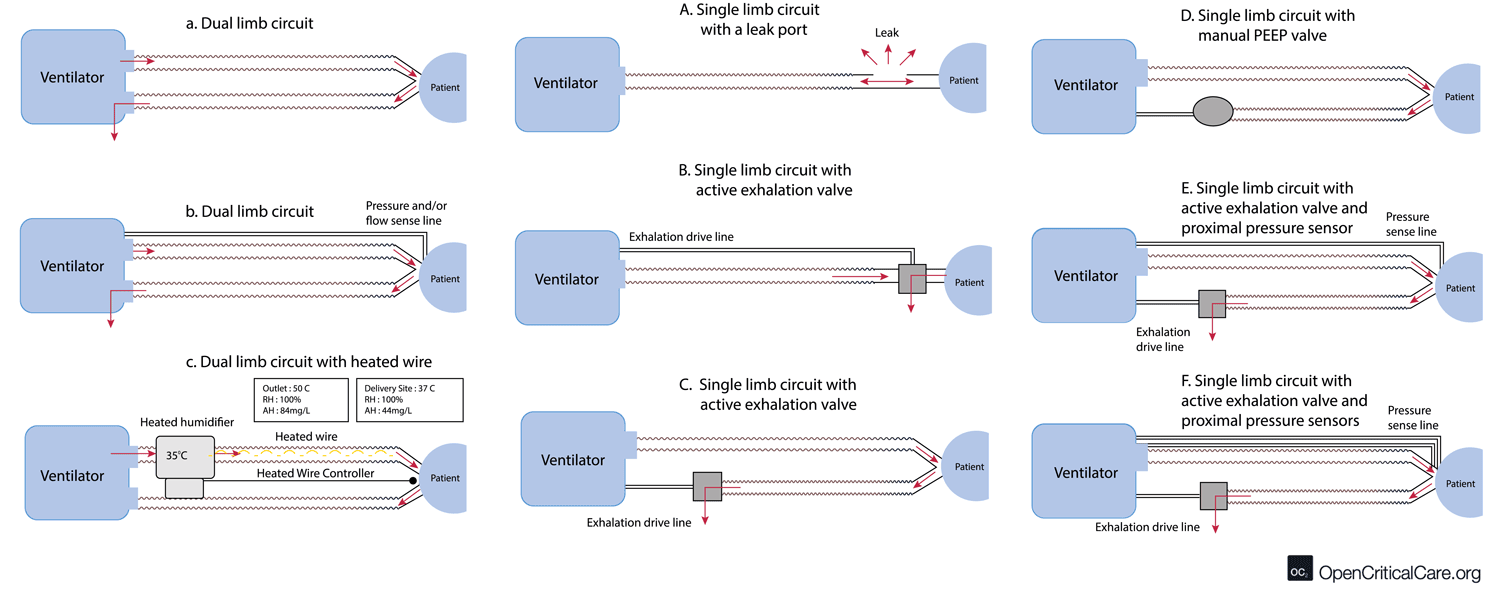
- There are multiple configurations of dual and single limb circuits (outlined below).
- Note on humidification & circuit configuration: some dual and single limb circuits may contain a heated wire in the inspiratory limb to optimize heat & humidification delivery to the patient and to prevent excess condensation from accumulating when using an active heated humidification system. If an active heated humidification system is used in the absence of a heated wire inspiratory limb, a water trap is often needed. Some water traps may allow for emptying without circuit disconnect (an important consideration with COVID19).
- Dual limb circuit (Figure a, b and c) – used by most traditional critical care ventilators. Flow/pressure and PEEP are commonly measured/controlled in the machine, and thus no additional circuit transducer tubing is needed (a). Some circuits do use proximal flow/pressure sensors (b). These may include a heating element in the inspiratory limb and port for temperature monitoring (c).
- Standard single limb with built in leak (figure A) – mostly for non invasive devices
- Standard single limb circuit with active exhalation valve and internal PEEP – (figure B and C) – These circuits are made by multiple manufacturers and can work with multiple vent models.
- Standard single limb circuit with active exhalation valve and manual PEEP – (figure D)
- Standard single limb circuit with active exhalation valve, internal PEEP and proximal pressure sensor – (figure E) – this is one of the most common single limb circuit setups
- Standard single limb circuit with active exhalation valve, internal PEEP and two proximal pressure/flow sensors – (figure F) – this is usually a proprietary circuit type that is commonly encountered and allows measurement of exhaled tidal volume
Pulse oximetry plays a key role in the diagnosis, triage and management of COVID-19 patients. This includes when to initiate hospitalization or oxygen therapy and when to escalate or deescalate oxygen therapy. This can be an essential tool for conserving oxygen supply as well.
Hypoxemia is a key finding in many patients with COVID-19. Up to 80% of patients with COVID-19 may have only mild symptoms or be completely asymptomatic. Common initial symptoms of COVID-19 infection include fever, cough, headache, fatigue, and myalgias. About 15% of infected patients develop severe disease, and about 5% develop critical illness – these patients may require supplemental oxygen. It has been reported that many patients with COVID-19 have more significant hypoxemia than their symptoms might suggest, which has been referred to as “happy” or “silent” hypoxia. Therefore, it is essential to check SpO2 for all patients with known or suspected COVID-19 infection who are symptomatic or report for medical evaluation.
References: Lifebox Pulse Oximetry Learning Module
Keywords: COVID-19, symptomatic, shortness of breath, SOB, coronavirus, oxygen
The optimal SpO2 for patients with respiratory failure has not been well established and is still being evaluated. The World Health Organization (WHO) guidance for patients with hypoxemic respiratory failure due to COVID-19 recommends the following targets: Initial SpO2 of >94% for stabilization, then >90% for stable patients who are not pregnant or 92-95% for stable patients who are pregnant. It is important not to make SpO2 goals too high, as this can cause oxygen toxicity and will deplete the oxygen supply more quickly. For more discussion on optimal SpO2 goals in patients with respiratory failure, please read more here.
References: WHO SARI Toolkit
Keywords: target SpO2, goal, respiratory failure, COVID-19
Conventional pulse oximeters assume that the major absorbers of light within arterial blood are deoxygenated hemoglobin (HHb) and oxygenated hemoglobin (O2Hb) and calculate SpO2 based on the ratio of measured absorbance of only two wavelengths of light (660nm and 940nm). Methemoglobinemia (MetHb) occurs when the iron in hemoglobin is oxidized, altering its ability to bind and offload oxygen.
The molar extinction coefficient of MetHb at 660 nm red light is approximately 1 and approximately equal to the molar extinction coefficient of HHb (see image below). The molar extinction coefficient of MetHb at 940 nm infrared light is also approximately 1 and significantly higher than the molar extinction coefficient of O2Hb. As a result of MetHb absorbing 660 nm and 940 nm light approximately equally, the Modulation Ratio (R-Value) approaches 1, this corresponds to an SpO2 of 80-88%. This can result in either an overestimation or an underestimation of SaO2 levels.
When a patient is cyanotic and methemoglobinemia is suspected, a co-oximeter capable of measuring the absorbance of greater than 2 wavelengths of light is necessary to determine fractional oxygen saturation and MetHb levels.
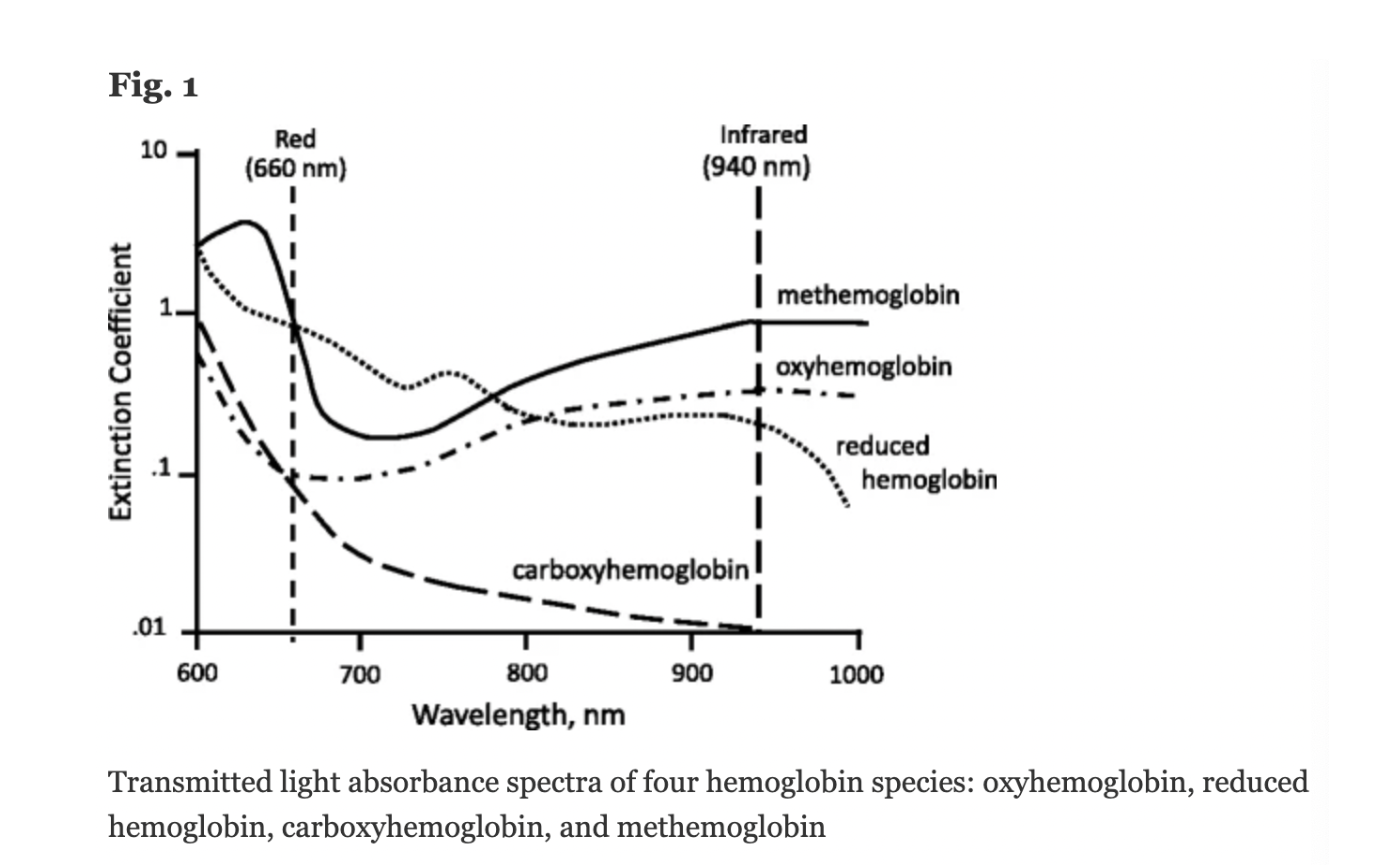
Figure: Jubran, Critical Care, 2015
Keywords: Dyshemoglobin, Methemoglobin, Methemoglobinemia,
References: Jubran, Critical Care, 2015 Chan et al, Respiratory Medicine 2013
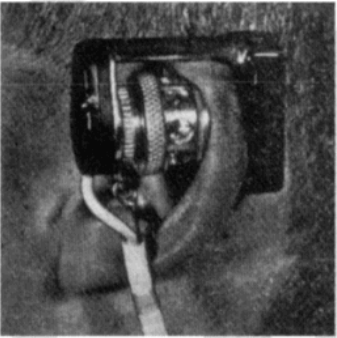
In 1940, J.R. Squire was the first to recognize that the differences in transmission of red and infrared light permitted oxygen saturation to be computed. In 1942, the British scientist Glen Millikan developed the first portable pulse oximeter to be used during pilot training in World War II. The device was placed on the earlobe and was used to monitor pilots’ oxygen saturation and safety during flight.
Thirty years later in 1972, the Japanese bioengineer Takuo Aoyagi developed conventional pulse oximetry by using the ratio of red to infrared light absorption in pulsating (arterial) blood to calculate oxygen saturation. This was later commercialized and gradually integrated into clinical practice in the 1980s and 1990s. Notably, the American Society of Anesthesiologists determined in 1986 that using pulse oximetry was to be recognized as the new standard of care. To learn more history about the development of the pulse oximeter, read here.
References: Severinghaus et al, J Clin Monit 1986; Millikan, Rev Sci Instrum 1942; Severinghaus, Anesth Analg 2007, Wood Library Museum of Anesthesiology
Keywords: first, invention, Millikan, Aoyagi
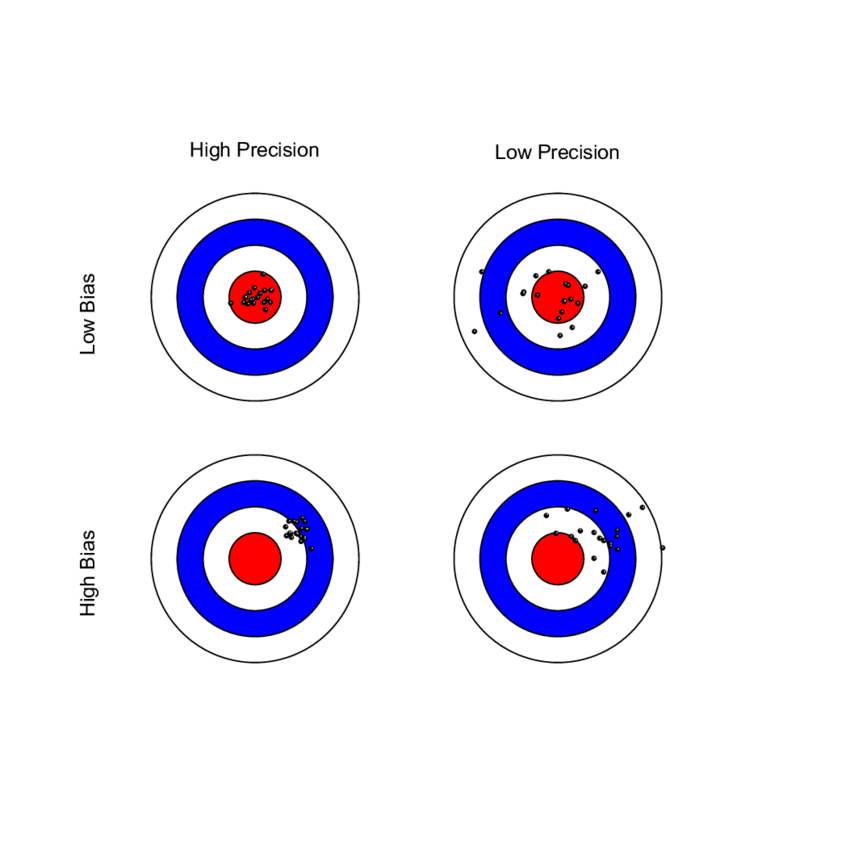
The Average Root Mean Square error or root mean square deviation (known as ARMS) is an established measure of SpO2 performance and is used by regulatory agencies to determine how accurately a pulse oximeter performs. Sometimes referred to as “Accuracy Root Mean Square error, ARMS is the square root of the mean of the squared deviations between the pulse oximeter SpO2 measurement and the SaO2 measurement. ARMS approximates the mean absolute deviation (MAD) between SpO2 and SaO2. It is always a positive value, so it cannot indicate whether SpO2 is negatively or positively biased for SaO2.
ARMS is calculated by combining both the bias and the standard deviation into one value. The FDA recommends an acceptable limit of ARMS ≤3% for transmittance devices and ARMS ≤3.5% for reflectance devices. If either component (bias or SD) is very large (i.e. inaccurate), the ARMS will be greater than the acceptable limit and will not be considered a well performing pulse oximeter. If a pulse oximeter device has an ARMS ≤3%, this means that it did not have a large scatter or large outliers in the data, and therefore can accurately measure SpO2. It is important to recognize that even a small difference in ARMS between devices can reflect a large difference in their performance.
ARMS is best referred to as a measure of performance (rather than accuracy).
References: FDA Guidelines; Clinical Application of ARMS in Pulse Oximetry – Clinimark June 2021; Brandmaier et al, Elife 2018
Keywords: bias, standard deviation, Arms, ARMS