Oxygen FAQ
Up to date, expert answers to frequently asked questions (FAQ) about oxygen supply systems, respiratory care and pulse oximetry written by OCC & collaborators.
Non-invasive ventilation (NIV)
- Most are not considered reusable per manufacturer specifications
- Check with the manufacturer specification and clinical guidelines to determine if reuse is safe
- Steps for disinfection must be closely adhered to and may be manufacturer specific
- Some reusable devices (e.g. some ventilator circuits) may have a finite lifespan (e.g. a predefined number of sterilizing cycles)
- Reusability of respiratory care devices is often debated and may vary based on local/national practice guidelines and regulations
Click here to review WHO tips for cleaning and disinfection of respiratory equipment
Additional resources:
- Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care (WHO)
- Disinfectants for COVID-19 (US EPA)
- Cleaning of CPAP and other devices used to administer supplemental oxygen (DPHSS Montana)
- Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. G. Kampf, D. Todt, S. Pfaender, E. Steinmann. Journal of Hospital Infection 104 (2020) 246-251.
- Disinfection and sterilization: an overview. Rutala, Weber. Am J Infect Control
- Disinfectants used for environmental disinfection and new room decontamination technology; Rutala, Weber. Am J Inect Control. 2013
- Guidelines for disinfection and sterilization in healthcare facilities, HICPAC, CDC 2019
- Reuse of anesthesia breathing systems: another difference of opinion and practice between the US and Europe, J Clin Anes 2008
- Bacterial and viral contamination of breathing circuits after extended use – an aspect of patient safety? Acta Anaes Scan, 2016
Most modern ventilators provide some form of NIPPV, however, there are significant differences in performance among devices. Much of the difference in performance is attributable to ability to compensate for large leaks (e.g. around the mask seal) and trigger latency. Newer ventilators may perform significantly better than older ventilators.
Any time the patient can cough or sneeze into the room – the risk of infection is increased. It is not clear that adding high flow nasal oxygen makes this worse. However, at a flow of 40-60 lpm if the patient coughs, model studies have shown some additional movement of particles in the room.
Healthcare worker infection from nosocomial transmission of SARS CoV-1was associated with lack of adequate infection control precautions especially in the presence of aerosol generating procedures. By definition this would also include HFNC. However, the risk of health care worker infection from HFNC was reported to be substantially less (8%) compared to intubation (35%) and NIPPV (38%) (Raboud et al. Plos One 2010). Moreover, placement surgical masks over a HFNC reduces the emission and dispersion of coronavirus bioaerosols (Leung et al. Nat Med 2020)
Any time the patient can cough or sneeze into the room – the risk of infection is increased. Some model studies show that if there is a mask leak – the high flow from the ventilator to compensate for the leak may force particles out into the room.
During the 2003 SARS CoV-1 outbreak, many healthcare workers became infected due to failure to implement adequate infection control precautions especially when performing aerosol generating procedures (Tran et al. PloS One 2012). However, because of the limited number of studies reporting NIPPV use in SARS CoV-1, the risk of infection could not be established with certainty. Moreover, in a Singapore study of SARS CoV-1, institution of rigorous protective measures (including air-purifying respirator units) for over 200 healthcare workers resulted in zero cases of infection (Lew et al JAMA 2003).
Ventilator connections vary but NIST connectors are common. Most ventilators are supplied with a high pressure hose with a NIST fitting on one or both sides and the other end of the hose with a fitting suitable to match the oxygen source (e.g. Shrader quick release)
Replace bacterial/viral filters as frequently as supplies allow in accordance with the manufacturer’s recommendations. This recommendation may be as often as every 24 hours, though the optimal interval may differ by setting and determined by assessing the risk:benefit of circuit disconnects, availability of supplies and ability to monitor for malfunctioning filters. Depending on location of the filter placement, circuit setup, humidification system and patient factors, B/V filters may function for multiple weeks, though this would be ‘off-label’ use. If an HME is used, a viral filter can be changed only with signs of increased resistance and may last a week or more. If a heated humidifier is used, the filter in the expiratory limb should be evaluated every 24 hours for signs of increased resistance and may need to be replaced every couple days, although this interval is highly variable. Always refer to the manufacturer’s recommendation. Lifespan may be significantly shortened if nebulized medications are being utilized or if copious secretions are present. Of note, disconnecting circuits can cause risk of aerosolization to healthcare workers.
Passive systems using Heat and Moisture Exchangers (HME) trap moisture and prevent it from being lost from the patient.
- Efficacy of these devices drop over time, causing increased resistance. Manufacturers may suggest a change every 24 hours, but studies have shown that an unsoiled device in some circumstances be used for several days or up to 1 week (Ricard et al, AJRCCM 2000; Thomacot et al, CCM, 2002; AARC. Resp Care. 2012).
- Signs of an increase in resistance include an increase in PIP but no change in Plateau pressure or a prolonged expiratory flow time.
- The most common cause of HME partial occlusion or rise in resistance is from pulmonary edema fluid or blood. Mucus generally clumps in a dependent portion of the device without increasing resistance appreciably. (Davis et al Crit Care Med. 2000)
Many would argue that ideally, breathing circuits should be changed between new patients though do not necessarily need to be changed on a routine basis for the same patient; change the breathing circuit only if it has been soiled or damaged (Han, Liu. Respir Care 2010).
However, it is important to note:
- there is considerable practice variation on this (especially prior to the pandemic and especially with anesthesia breathing circuits); often depends on local/national regulations and patient specific factors
- circuit change practices have considerable impact on the environment
Additional Readings:
This is somewhat controversial.
For short periods of time (hours), NIPPV may be tolerated without humidity, but for longer periods of time (days) humidity is essential, especially at high FIO2 (100% oxygen from a tank or other high pressure source is ~anhydrous). Leaks with NIPPV (around a mask seal) create issues with humidification and is one reason HMEs don’t work well with NIPPV.
For HFNC, most people find >6LPM NC intolerable without humidification, but with humidification most people don’t notice flow until it exceeds 15LPM and will tolerate >40LPM. With HFNC (and nasal CPAP) gas flow is unidirectional (in the nose and out the mouth) and thus there is no possibility for reclaiming moisture from the exhaled breath. Without heat and humidification most patients will find this intolerable.
Flow triggering is a popular method for allowing patients to initiate breaths during mechanical ventilation. It works by setting a continuous “background flow” of gas through the ventilator circuit during expiration called Bias Flow. During spontaneous breathing inspiratory effort diverts some of this background flow away from the circuit to the patient. This resulting decrease in background flow exiting the ventilator is sensed and triggers an assisted breath.
Settings: The clinician sets the flow trigger sensitivity as they would pressure sensitivity. This is typically set at 1-2 L/min below the bias flow setting which may be programmed into the ventilator or can be adjusted by the clinician (e.g. 5-20 L/min). Bias flow can be a significant source of oxygen consumption.
Not all ventilators use bias flow and some have different methods of achieving flow triggering. Always refer to the product manual for device specifications.
Issues Related to Flow Triggering
Circuit Leak: If a leak develops in the circuit gas flow will be diverted away from the expiratory flow sensor causing ventilator malfunction by continuous triggering (“chattering” ) analogous to an excessive sensitivity that will impede ventilation and set-off an alarm condition.
Inappropriate Sensitivity Setting: An insensitive flow trigger level (e.g. > 5 L/min below bias flow) will increase the time delay between the onset of patient effort and the onset of the mechanical breath that often results in substantial increases in patient work of breathing and respiratory drive and patient-ventilator asynchrony. As described above, a too sensitive ventilator causes excessive triggering that can interfere with ventilation and can increase the risk of hyperinflation, barotrauma, hemodynamic instability and hyperventilation during assist-control or high levels of pressure support ventilation.
Artifacts during Indirect Calorimetry: Bias flow through the circuit interferes with the measurement of VO2 and VCO2 as it increases the total ventilation measurement by the calorimeter above the patients minute ventilation. Pressure-trigger should be used during calorimetry measurements.
Inadvertent PEEP: In some of the older ventilators that allow a high bias flow (e.g. 15-20 L/min), these flows may interact with the resistance of the expiratory block (pneumotachographs and valving) to create circuit backpressure. This would be more prominent when higher levels of PEEP are used in tandem with high bias flow. This can be easily detected when PEEP measured within the circuit exceeds the set PEEP (e.g. set PEEP = 10 and measured PEEP = 14 cmH2O).
Example Bias flow O2 consumption calculation:
For the following scenario, total oxygen consumption and oxygen consumption due to bias flow are 16.7 LPM and 6.7 LPM respectively. RR 20, TV 500, bias flow 10, leak zero, FiO2 1.0, expiratory time 2.
[Device O2 consumption rate in LPM = (Minute ventilation + (bias flow x RR x expiratory time/60) + leak) x (FiO2 – 0.21)/0.79]
Additional resources:
Ventilator Bias Flow Value Database (Coming soon)
Calculator to estimate impact of bias flow on O2 consumption
- It depends on the device setup. Ventilators may require ‘external’ filters (viral, HME, and fan, as well as air intake filters for turbine or compressor ventilators) and internal filters (oxygen inlet filter). Filters can provide three kinds of functions:
-
- Filtering particulate matter
- HEPA (high-efficiency particulate air) filters rated to 3 microns are generally considered ‘acceptable’ for bacterial and viral filtration. The term HEPA refers to the efficiency of capturing particles with a MPPS (most penetrating particle size) diameter of 0.3 microns.
- Of note, machines that accept 50psi/4bar gas intake usually have internal bronze sintered filters to protect the machine from contaminated gas sources. On occasion additional external filters on the high pressure gas lines are required to prevent damage to the device.
- Preserving heat and moisture
- Heat and Moisture Exchangers (HME) are commonly rated to 3 microns, the HEPA standard (and may be referred to as HME filters – HMEF), but may not be. They are composed of foam, paper or other material that allows moisture to condense.
- Hygroscopic Condenser Humidifiers (HCH) are functionally the same as HMEs, though have a slightly different mechanism as they are impregnated with a salt to aid in moisture condenstation. In the past, HME types were separated based on the additional treatment of the media. An HME relied solely on physical principles (the earliest ones were all aluminum) whereas the HCH included treatment of the media with a hygroscopic salt (LiCl, now mostly CaCl). At present, the reality is that most HME devices also take advantage of the hygroscopic salt (check manufacturer’s specifications to confirm this). In theory, if one were to push the media out of an HME then touch it to the tip of the tongue – it would be very salty (DO NOT TRY THIS).
- The moisture efficiency of an HME or HCH is dependent on its size, media density and salt treatment.
- HMEs may be referred to as type I (adult) and type II (pediatrics), which differ in deadspace and functional tidal volume range.
- Unless specifically designated as having capacity for ‘filtration’ (HMEF), HMEs do not provide adequate filtration of bacteria and viruses.
- Filtering bacteria and viruses
- Bacterial/viral (B/V) filters are defined by the ability to filter particles with a diameter size of 3 microns though may be rated to particles as small as 0.2 microns, and do not necessarily provide heat/moisture preservation.
- The minimum viral filtration efficiency (VFE) that is needed to ensure SARS COV-2 virus can not pass from the patient to the room or machine is unknown.
- B/V filters with 99.97% ASTM efficiency or filters with >95% efficiency for MPPS of 0.3 microns may be recommended to prevent SARS-CoV-2 viral transmission though data and standards continue to evolve.
- Diameter for SARS-CoV-2 virion is ~0.06-0.14 microns, hepatitis C is 0.03 microns and Staph aureus is 1 micron.
- Filtering particulate matter
- Combination filters providing all three functions are available. Filters that conserve heat and moisture and provide B/V filtering are often referred to as HMEF.
- More info on filter types and efficiency testing
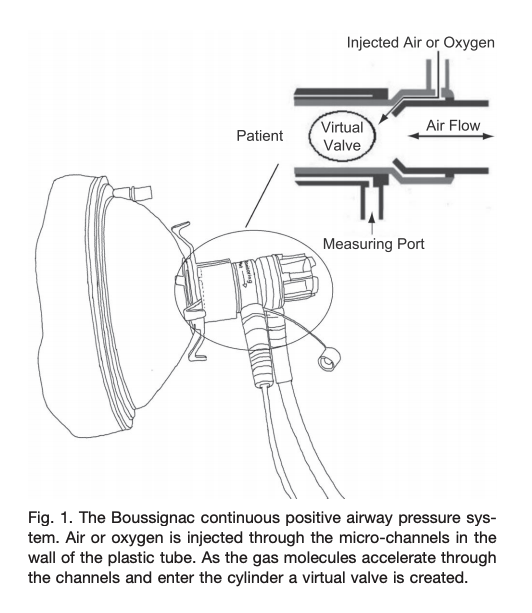
- Boussignac is a low cost, easy to use facemask CPAP-like system that has no sensors, mechanical valves or electrical components. The system connects to an oxygen flowmeter/source that generates flow dependent pressure (8LPM ~3cmH20; 15LPM ~5cmH20; 23LPM ~10cmH20)
- Traditionally used by prehospital providers for cardiogenic pulmonary edema
- In patients with hypoxemic respiratory failure (non cardiogenic pulmonary edema), it is unclear if this device would work well in patients with high minute ventilation (Sehlin et al, Resp Care, June 2011).
- There are several noteworthy potential limitations:
- Utilizes high oxygen flows
- Limited ability to adjust FiO2
- At high flows will cause airway dryness and discomfort.
- No leak compensation
- No monitoring of pressures
It depends on several factors. Not all masks, circuits or devices are compatible or able to deliver CPAP/NIPPV.
The basis of non-invasive CPAP/NIPPV is a constant gas flow against an expiratory resistor that creates positive “back pressure” in the circuit (i.e. Pressure = Flow x Resistance). Back pressure is increased either by increasing gas flow against the resistor or increasing the resistance to gas flow.
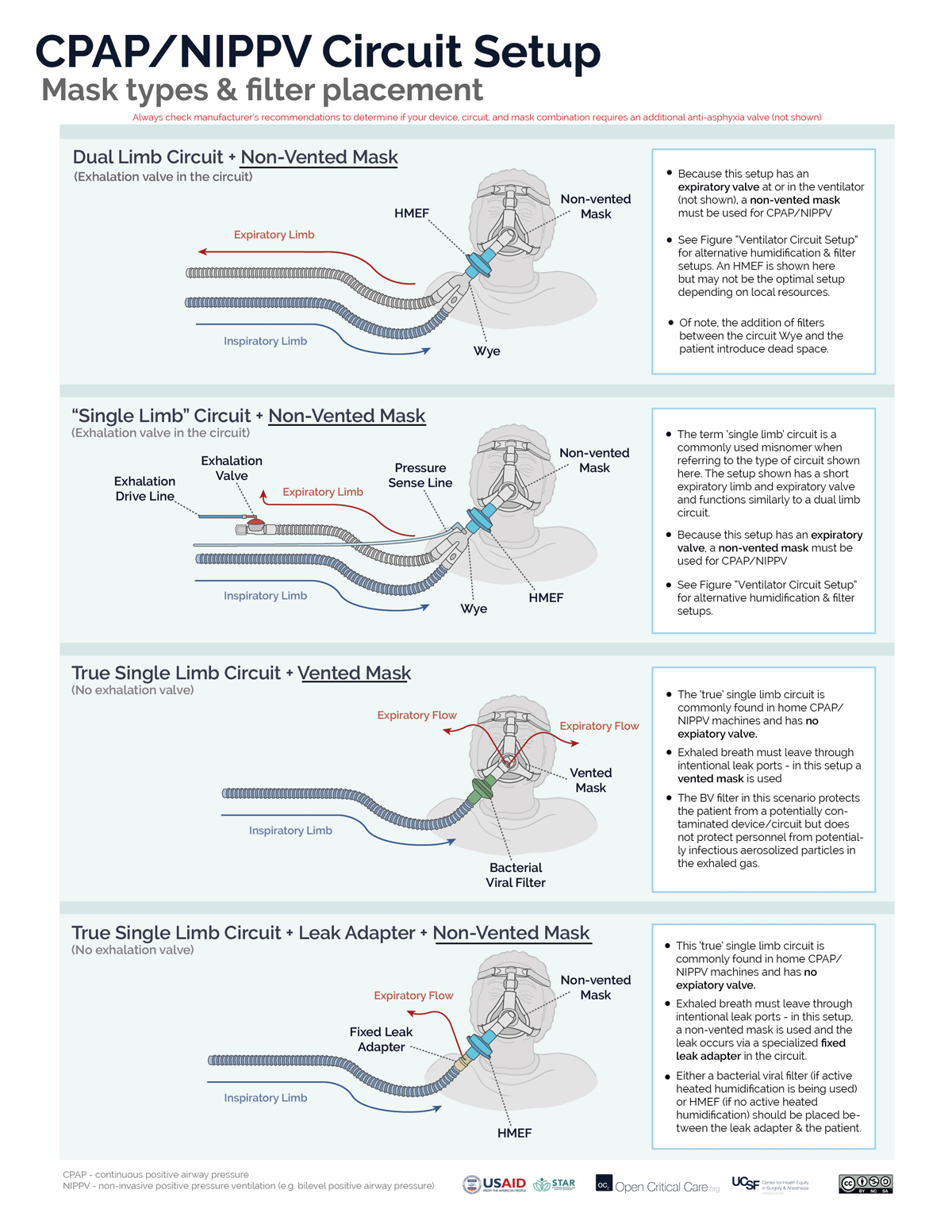
There are 2 circuit options for non-invasive CPAP (or NIPPV/BiPAP):
- A “true” single limb circuit (commonly found in home NIPPV/BiPAP devices) has 1 hose directing gas from the device to the mask and no active expiratory valve. There are two common setups for NIPPV/CPAP with a true single limb circuit. (Note: circuits with both an inspiratory limb and a short expiratory limb with expiratory valve, while commonly referred to as ‘single limb’ are not ‘true’ single limb circuits)
- “Vented Mask” setup – The exhaled breath has to get out of the patient/circuit somewhere, therefore, the mask must act both as the resistor and the exhalation port. A vented mask has holes that allow for fixed resistance. Because the resistance is fixed, CPAP is increased by increasing flow through the circuit.
- Fixed leak valve circuit setup – An alternative option to using a vented mask, is to use a non-vented mask and to place the fixed/intentional leak into the circuit by adding a ‘leak valve,’ which contains holes that allow fixed resistance.
- A dual limb circuit (commonly found in ICU and transport ventilator) has 2 hoses connected by a “Y” (or “wye”) adapter where the expiratory limb directs gas flows to an active expiratory valve that controls expiratory resistance. The expiratory valve either is clearly visible in the expiratory hose (“expiratory manifold”) or the expiratory hose attaches directly back into the ventilator (i.e. the expiratory manifold is built into the ventilator). To function properly (ie to create/adjust CPAP, or pressure-support/ BiPAP) the circuit must only be used with a non-vented mask (i.e. the ventilator will control both gas flow and resistance).
- Troubleshooting Note: Using a vented mask with any dual limb ventilator circuit will result in the inability to effectively create sufficient positive pressure, increased aerosolization and likely set-off alarms.
Some ventilators can utilize both true single limb and dual limb setups. Another approach to determine the correct mask type for NIPPV/CPAP is to determine if the system (ventilator or circuit) has an an active exhalation valve:
- System with active exhalation valve – non-vented mask required
- System without active exhalation valve – vented mask required (or fixed leak adapter in the circuit)
When access to optimal equipment is not readily available, alternative solutions (https://link.springer.com/article/10.1007/s41782-020-00092-7) have been proposed and reported with uncertain outcomes. These are most often not in line with manufacturers’ recommendations and are not endorsed here. Always check manufacturer recommendations for all devices to confirm correct equipment and mode settings.
Continuous Positive Airway Pressure (CPAP) devices or modes apply constant pressure throughout the respiratory cycle via face mask or other interface to splint open the upper airway, increase lung volume, and increase intrathoracic pressure. CPAP provides no inspiratory muscle unloading and tidal ventilation remains completely dependent on the respiratory muscles.
Non-invasive ventilation (NIV) or Non-invasive positive pressure ventilation (NIPPV) applies two levels of pressure during the respiratory cycle – a pressure during the inspiratory phase that is greater than the pressure applied during exhalation. This is effectively mechanical ventilation, and can unload the respiratory muscles and provide complete respiratory support.
Bilevel positive airway pressure (BIPAP) is a branded/trade name (by Phillips) for NIPPV/NIV as described above
Estimated Fraction of Inspired Oxygen (FiO2)
Nasal Cannula
1
0.24
-
2
0.28
-
3
0.32
-
4
0.36
-
5
0.40
Simple Facemask
6-10
0.44-0.50
Non-Rebreather Mask with Reservoir
(reservoir must be fully inflated)
10-20
Approx 0.6-0.8
At RR ~20 & Tidal Volume ~500
20 LPM flow = ~60% FiO2
30 LPM flow = ~70% FiO2
40 LPM flow = ~80% FiO2 (Farias et al).
The values represent estimates of FiO2. Actual FdO2 (delivered O2 concentration) is dependent on multiple factors including oxygen supply quality, patient’s minute ventilation and inspiratory flow rate. One general estimation rule is using oxygen flow rate: FiO2 =0.21 + 0.03 x oxygen flow rate in L/min (Frat et al).
It depends on the positive pressure device and circuit being used. If a single limb circuit without an active exhalation valve is being used (e.g. a home NIPPV machine), then a vented mask or vented circuit must be used (i.e. fixed orifice resistor outlet). If a dual or single limb circuit with an active exhalation valve is being used, then an NIV mask without a vented port should be used. Read more on circuit and mask setup for NIPPV/CPAP

Moisture present in patients’ lungs is rapidly lost at high breathing rates.
When breathing dry air, cilia stop functioning properly (in a matter of hours – Hirsch et al J Appl Physio 1975). When the moisture level becomes low, mucous in the patient’s lungs can become thick and hard, and quickly block the patient’s airways, or the endotracheal tube, stopping airflow.
Additionally, heat is rapidly lost to non-humidified air.