Oxygen FAQ
Up to date, expert answers to frequently asked questions (FAQ) about oxygen supply systems, respiratory care and pulse oximetry written by OCC & collaborators.
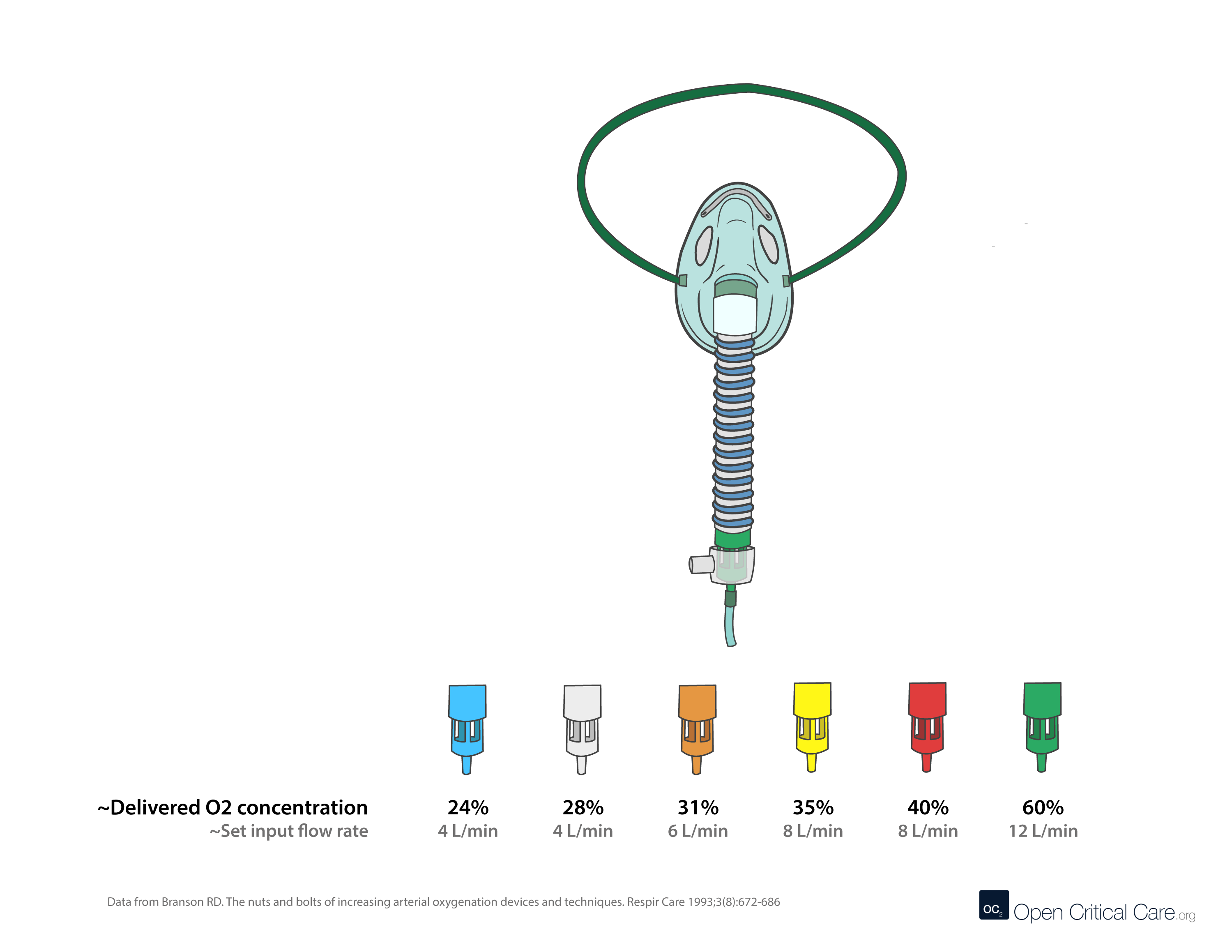
Air entrainment mask
- Most are not considered reusable per manufacturer specifications
- Check with the manufacturer specification and clinical guidelines to determine if reuse is safe
- Steps for disinfection must be closely adhered to and may be manufacturer specific
- Some reusable devices (e.g. some ventilator circuits) may have a finite lifespan (e.g. a predefined number of sterilizing cycles)
- Reusability of respiratory care devices is often debated and may vary based on local/national practice guidelines and regulations
Click here to review WHO tips for cleaning and disinfection of respiratory equipment
Additional resources:
- Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care (WHO)
- Disinfectants for COVID-19 (US EPA)
- Cleaning of CPAP and other devices used to administer supplemental oxygen (DPHSS Montana)
- Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. G. Kampf, D. Todt, S. Pfaender, E. Steinmann. Journal of Hospital Infection 104 (2020) 246-251.
- Disinfection and sterilization: an overview. Rutala, Weber. Am J Infect Control
- Disinfectants used for environmental disinfection and new room decontamination technology; Rutala, Weber. Am J Inect Control. 2013
- Guidelines for disinfection and sterilization in healthcare facilities, HICPAC, CDC 2019
- Reuse of anesthesia breathing systems: another difference of opinion and practice between the US and Europe, J Clin Anes 2008
- Bacterial and viral contamination of breathing circuits after extended use – an aspect of patient safety? Acta Anaes Scan, 2016
Air-entrainment (‘Venturi’) masks can consume vast quantities of oxygen. They are designed to be used when precise control of FiO2 is needed (e.g. patients with dependence on hypoxic ventilatory drive like some COPD patients). The table below shows flow set on the Venturi (Air entrainment mask) – the total flow (O2 flow + air entrained) and the FIO2. This helps demonstrate why at low FIO2 – the Venturi mask provides a far more consistent FIO2 than nasal cannula or simple facemask. However, it should be noted that relatively high flows of 100% O2 are required to achieve high FiO2.
0.24
4
25:1
104
0.28
4
10:1
44
0.31
6
7:1
48
0.35
8
5:1
48
0.40
8
3:1
32
0.50
12
1.7:1
32
0.60
12
1:1
24
0.70
12
0.6:1
19
Estimated Fraction of Inspired Oxygen (FiO2)
Nasal Cannula
1
0.24
-
2
0.28
-
3
0.32
-
4
0.36
-
5
0.40
Simple Facemask
6-10
0.44-0.50
Non-Rebreather Mask with Reservoir
(reservoir must be fully inflated)
10-20
Approx 0.6-0.8
At RR ~20 & Tidal Volume ~500
20 LPM flow = ~60% FiO2
30 LPM flow = ~70% FiO2
40 LPM flow = ~80% FiO2 (Farias et al).
The values represent estimates of FiO2. Actual FdO2 (delivered O2 concentration) is dependent on multiple factors including oxygen supply quality, patient’s minute ventilation and inspiratory flow rate. One general estimation rule is using oxygen flow rate: FiO2 =0.21 + 0.03 x oxygen flow rate in L/min (Frat et al).