Oxygen FAQ
Up to date, expert answers to frequently asked questions (FAQ) about oxygen supply systems, respiratory care and pulse oximetry written by OCC & collaborators.
Top 10 most popular FAQs
Elevated bilirubin has been reported by some studies to potentially underestimate oxygen saturation by pulse oximetry, though most available data have not shown any effect on the accuracy of Spo2 with bilirubin levels up to 84.3 mg/dlitre. The light absorption spectrum of bilirubin has a broad peak at 460 nm and two smaller peaks at 560 nm and 600 nm. Based on this, bilirubin is relatively unlikely to have a detectable effect on the absorption of the 660 nm and 940 nm wavelengths commonly used by pulse oximeters.
References:
https://pubmed.ncbi.nlm.nih.gov/2024749/
https://pubs.asahq.org/anesthesiology/article/70/1/118/30895/Hyperbilirubinemia-Does-Not-Interfere-with
https://pubmed.ncbi.nlm.nih.gov/2967958/
‘Differential bias’ (sometimes referred to as disparate bias) attempts to describe how much a pulse oximeter performs differently in people with light skin pigment as compared to dark skin pigment. To determine this, we compare the device’s bias (how much it over or under-reads SpO2) for people with very light skin and people with very dark skin*.
Differential bias is calculated independently for two different SpO2 ranges (70-85% and 85-100%). Thresholds for allowable differential bias are not yet established though proposals have targeted 3.5-4% for the SpO2 70-85% range, and 1.5-2% for the SpO2 85-100% range.
For example:
- Scenario 1: If a pulse oximeter on a person with light skin reads 1% higher than their true blood oxygen saturation (e.g., the oximeter shows 91% when the actual blood oxygen saturation is 90%), and on a person with dark skin it reads 2% higher (e.g., it shows 92% when the actual blood oxygen saturation is also 90%), the differential bias between the two is 1%.
- Scenario 2: If the pulse oximeter on a person with light skin reads 2% lower than their true blood oxygen saturation (e.g., the device shows 88% when the actual blood oxygen saturation is 90%), and on a person with dark skin it reads 1% lower (e.g., it shows 89% when the true value is 90%), the differential bias here is also 1%, but in the opposite direction.
- Scenario 3: If the pulse oximeter on a person with light skin shows no bias (e.g., reads exactly 90% when the actual blood oxygen saturation is 90%), but on a person with dark skin it reads 5% higher (e.g., the device shows 95% when the actual value is 90%), the differential bias is 5%.
These examples demonstrate how differential bias can manifest as either higher or lower readings depending on skin pigment.
*Using modeling of skin pigment and SpO2 data, very light skin and very dark skin are defined as having an difference in ITA of 100. Read more about ITA here.
When you click on a device to view its standard performance details, some of the terms can be unfamiliar. To help, we’ve put together this guide to explain some of these terms and concepts.
NOTE: If you come across something that isn’t explained here, you can simply hover over the dark grey “i” button for a quick explanation. If you’re still unsure, feel free to contact us, and we’ll clarify!
——————————————————————————————————————————————————–
How do we determine how accurate the oxygen saturation measured by the pulse oximeters is?
Pulse oximeters estimate oxygen saturation (SpO2), which is the percentage of hemoglobin in the blood that is bound to oxygen. Pulse oximeters do this non-invasively by shining light through the skin. The most accurate way to measure oxygen saturation, however, involves taking a blood sample from an artery and analyzing it with a specialized device called a blood gas analyzer.
To assess the accuracy of pulse oximeters, we compare the oxygen saturation from the pulse oximeter to the oxygen saturation from the blood gas analyzer. We do this in healthy adults, in a controlled lab setting, by gradually and safely lowering their oxygen levels from a saturation of 100% down to 70%. Specialized statistical methods like ‘ARMS’ (described elsewhere in this FAQ) are then used to evaluate the pulse oximeter’s performance. For more information about our process, please refer to our study protocol.
——————————————————————————————————————————————————–
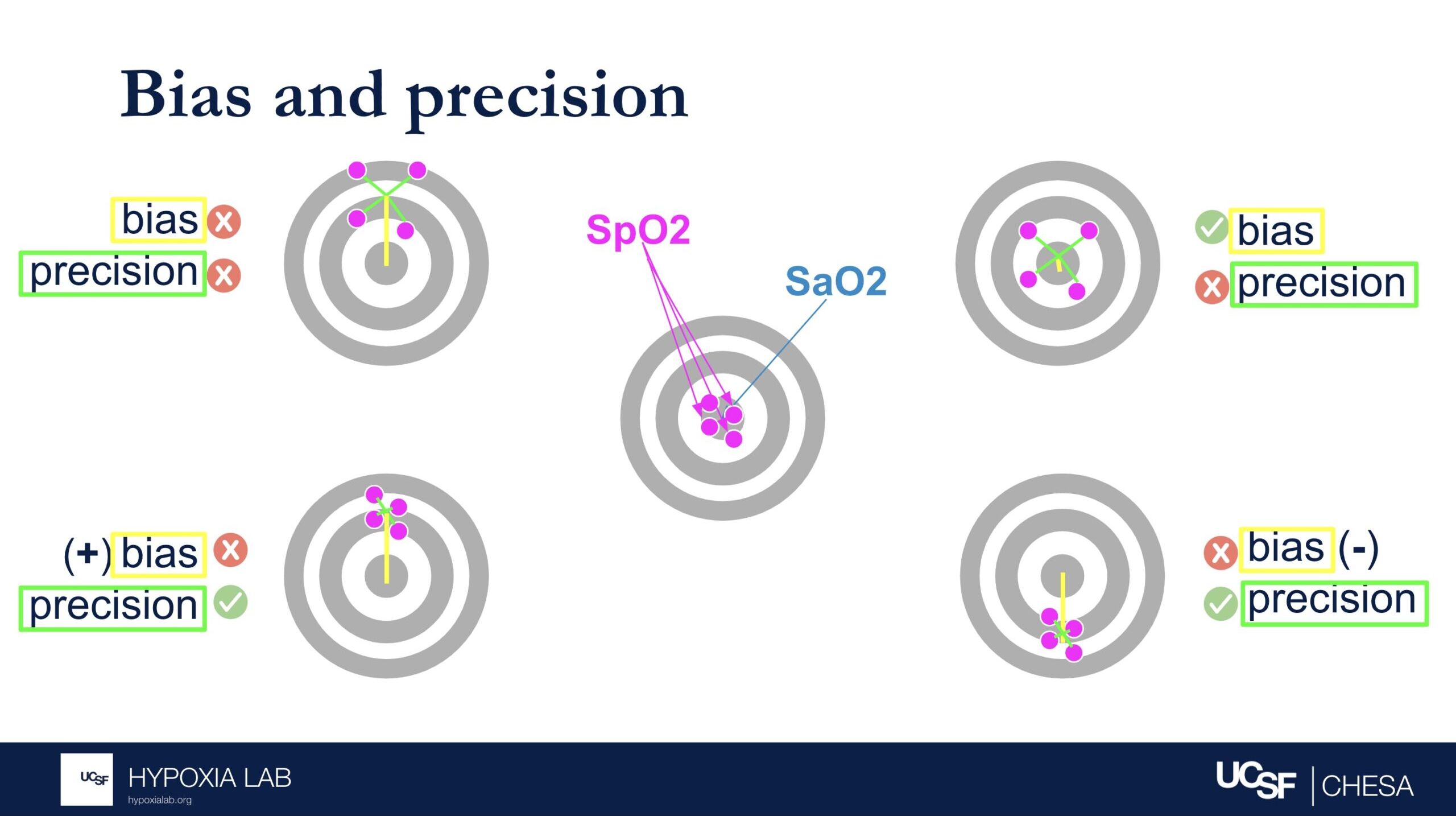
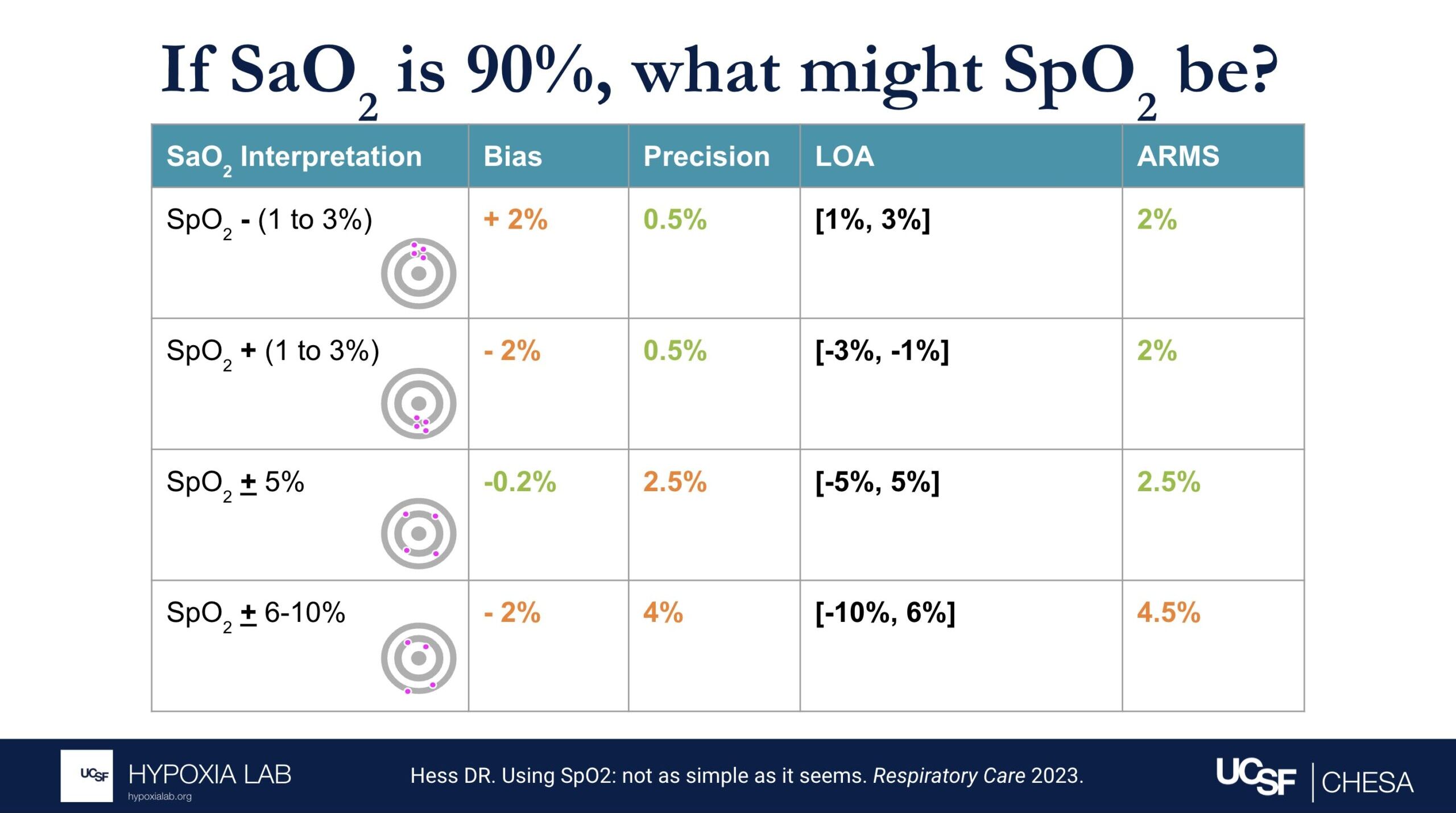
Can you explain what the manufacturer-claimed ARMS (Root Mean Square Error) for the SpO2 range of 70-100% refers to?
The ARMS can be a confusing term to describe pulse oximeter performance, but it is the most common metric used and is required by regulatory bodies. ARMS stands for accuracy root mean square. It tells you, on average, how close the device’s readings are to your true blood oxygen levels, when the oxygen levels are between 70% and 100%, based on tests in healthy adults in controlled laboratory studies.
It’s helpful to understand that a lower ARMS means the device readings are more accurate and precise. For example, with an ARMS of 2%, the device’s readings would typically be within approximately 2-4% of the true oxygen level. So, if your true oxygen saturation is 94%, then the pulse oximeter reading may be between 90% and 98%.
Under the standard performance information for a particular device, there are two different ARMS values listed: 1) An ARMS that’s reported in the product manual from the manufacture (Manufacturer claimed Arms), 2) The Independent ARMS that we calculate from our testing here at the UCSF Hypoxia Lab using our study protocol.
We follow current FDA guidance and ISO standards for testing and also add more elements to our testing protocols to better account for diversity of multiple factors like different skin colors and different levels of blood flow to the hands (factors that may impact performance of pulse oximeters).
For more information about ARMS, check out this detailed FAQ.
——————————————————————————————————————————————————–
What does the “Independent ARMS Study Cohort Size” mean?
This refers to how many healthy adults were tested using the OpenOximetry.org Protocol for this particular device. We share preliminary results after testing in at least 10 people (which is the minimum number required by the FDA guidance and ISO standards as of October 2024), but we continue testing the devices on as many different participants as possible to improve the diversity of people included. We are also waiting for updated guidelines to know the best number of participants for these tests.
——————————————————————————————————————————————————–
How is skin pigment defined?
Skin pigment refers specifically to the amount and type of melanin in your skin. People with darker skin have more melanin, while those with lighter skin have less.
We’re working hard to understand how skin pigment affects pulse oximetry readings. To learn more about how we measure skin pigment in our studies, check out our Skin Color Quantification page and our FAQ on skin pigment.
——————————————————————————————————————————————————–
Why might openoximetry.org results be different from what a manufacturer or other testing has reported?
While our testing follows FDA guidance and ISO standards for evaluating pulse oximeter performance, some aspects of our procedures may differ from those used in other testing labs. For instance, we test devices on a different group of people than those used by other labs, and device performance can vary between groups. Additionally, one way our testing may differ, while still adhering to ISO standards and FDA guidelines, is in how we modify participants’ physiology. For example, in pulse oximeter testing on the fingers, it is common practice (and allowed under current regulations as of October 2024) to warm participants’ hands to improve circulation and enhance the signal. This may result in better performance for some devices during warming than without warming. However, for the Open Oximetry Project, we do not routinely warm participants’ hands during testing.
——————————————————————————————————————————————————–
Why might openoximetry.org results change over time?
Our results will continue to evolve as we continue testing devices in more people, learning more about how to best measure performance, and as regulatory recommendations for testing change. This is an ongoing process, and the science is still developing, so it’s not perfect yet. There are many factors that affect how pulse oximeters perform, and we can’t account for all of them with the current testing protocols. As we collect more data from a wider range of devices and diverse groups of people, our understanding and results may change. So, it’s a good idea to check back regularly for updated information!
——————————————————————————————————————————————————–
What does pulsatility amplitude mean?
Pulse oximeters look for the pulsatile (i.e. beating or changing) signal coming from the blood vessels to measure oxygen levels in the blood. Some devices report the pulsatility in various ways like perfusion index, pulsatility amplitude, percent modulation or other ways. This gives us an idea of how strong the blood flow signal is in the area being measured. For more information, check out our FAQ on perfusion.
——————————————————————————————————————————————————–
What is differential bias?
Differential bias (sometimes called disparate bias) refers to how much a pulse oximeter’s performance can vary between people with light skin and those with dark skin. To measure this, we compare how much the device over- or under-estimates blood oxygen levels (SpO2) in people with very light skin versus those with very dark skin*.
*Using modeling of skin pigment and SpO2 data, very light skin and very dark skin are defined as having an difference in ITA of 100. Read more about differential bias here.
——————————————————————————————————————————————————–
What is lifetime cost?
*CAUTION: Our Lifetime Cost Calculator is a beta feature, makes many assumptions, is not based on the specific manufacturer/model durability, and costs may not reflect cost of ownership in well-resourced or home care settings. Costs are based solely on the purchase cost for this oximeter and general assumptions about how long a device with this form factor (i.e., fingertip vs handheld vs tabletop) might be expected to last in a heavy-use environment (e.g., a resource-variable clinical setting with frequent use, where damage, loss and theft are possible). These assumptions were informed by input from clinicians across diverse settings. Lifetime ownership cost varies considerably by user and setting. Click the settings button next to the cost to view the formula and adjust these assumptions based on your local context and use case.
▪ Two antiviral medications are effective and approved for Test to Treat: Paxlovid (nirmatrelvir/ritonavir, or NMV/r) and Lagevrio (molnupiravir, MOL). These are intended to be used in outpatient (non-hospitalized) settings. Generic versions of these medications are anticipated in upcoming months – continue to be attentive to local guidelines regarding specific details and availability.
▪ There are other treatments indicated for patients with more severe disease requiring advanced levels of care (i.e., treatment in a hospital due to severe or critical COVID-19). The goal of oral antiviral therapy is to reduce the risk of requiring an advanced level of hospital care and reduce the risk of death.
-By EPiC FHI360
▪ Symptomatic COVID-19 patients, confirmed with a positive test, within five days of onset of symptoms, and who are at risk for progression to severe disease.
▪ Patients must be age 12 or older and weigh at least 40 kilograms (88 pounds) to take NMV/r, and age 18 years or older to take MOL, but generally, the Test to Treat strategy is geared to adult patients with risk factors for developing complications.
▪ Risk factors for developing severe or critical COVID-19 include (but not limited to): Older than age 50; risk increasing substantially at age 65 and above; Chronic medical diseases such as pulmonary/lung disease, hypertension, diabetes, chronic kidney disease, immunocompromised state, HIV infection, obesity (BMI > 30kg/m2)
▪ See treatment algorithm and other resources for more details
-By EPiC FHI360
▪ It is important to reconcile or update a patient’s current medication list prior to starting oral antiviral therapy. There are important drug-drug interactions (especially for NMV/r), and there are several resources to guide dosing adjustments and/or interruptions as a patient completes their course of antiviral therapy (for example https://www.covid19- druginteractions.org/).
▪ The dosage for Paxlovid is 300 mg nirmatrelvir (two 150 mg tablets) with 100 mg ritonavir (one 100 mg tablet), with all three tablets taken together orally twice daily for five days. The full five-day course should be completed in conjunction with continued isolation according to public health recommendations.
▪ The dosage for Lagevrio (molnupiravir) is 800 mg (four 200 mg capsules) orally, every 12 hours for five days. The full five-day course should be completed in conjunction with continued isolation according to public health recommendations.
▪ The drugs can be taken with or without food. The tablets or capsules should not be cut, crushed, or broken. ▪ Drug-drug interactions should be considered; specific details and recommendations for dose adjustments can be found on the Test to Treat antiviral therapy algorithm
-By EPiC FHI360
▪ Consensus does not exist on the recommendation of NMV/r for pregnant patients. The US FDA and NIH state that for a mother and unborn baby, the benefit of taking NMV/r may be greater than the risk from the treatment, given existing animal studies and the extensive use of ritonavir in pregnant women with HIV. By contrast, WHO states that their strong recommendation for its use does not apply to pregnant patients. Decision making regarding prescription of NMV/r should be made in consultation between the patient and the health care worker, considering specific risks and benefits.
▪ Molnupiravir should not be used in pregnancy, and both men and women should be counseled to use a reliable method of contraception to avoid pregnancy within four days (females) and three months (males) of completing the course of molnupiravir.
-By EPiC FHI360
▪ For those who are eligible, discuss the benefits, efficacy, and goals of treatment, along with standard counseling on common and rare side effects when prescribing any medication.
▪ For those who are NOT eligible, explain why they do not qualify for a prescription. Some patients may feel confused or upset if they cannot have the treatment for COVID-19. Reassure them that the health care team is providing the best, evidence-based treatment and care even if it cannot include oral antivirals. Any member of the health care team taking care of low-risk patients who are not eligible for oral antivirals can explain that oral antivirals are only indicated for people at risk for developing complications that may lead to death. Oral antivirals have not been shown to reduce illness severity in low-risk patients, and benefit has not been demonstrated in people at-risk who start treatment more than five days after symptom onset. Supportive symptom management at home will likely have the same therapeutic effect without the concerns for side effects of the medication.
▪ Counsel patients to remain in isolation through the course of treatment (5 days after diagnosis); After day 5, continue isolation if still having significant symptoms or requiring medication for fever and symptom control.
▪ Counsel patients about basic supportive care (i.e., rest, hydration, nutrition, analgesia, antipyretics, etc.) and about the typical progression of mild or moderate COVID-19.
▪ Tell patients if their symptoms worsen to contact a health care provider or return to the clinic for further evaluation.
▪ Counsel patients to contact the health care team if rebound symptoms occur. Rebound symptoms have been reported but are usually mild. If COVID-19 symptoms return after completion of the oral antiviral course, consider repeat testing and have the patient continue to isolate if still testing positive for COVID-19. A repeat course of oral antivirals is not indicated for rebound symptoms.
▪ If patients live with other people, consider potential exposure to those individuals and their possible risk factors. Encourage patients and families to remain vigilant and test liberally
-By EPiC FHI360
▪ The World Health Organization recommends the use of oral antivirals as part of the Living Guidelines for Treatment of COVID-19. Opencriticalcare.org features both a Test to Treat Algorithm (for clinical management) and Implementation Guide for workflows in clinical settings.
▪ Consult other training materials made available as part of this Test to Treat strategy.
-By EPiC FHI360
Anyone with symptoms should be tested, even if symptoms are mild. Symptoms include fever, cough, fatigue, loss of taste and smell, shortness of breath, sore throat, runny nose/congestion, body aches/muscle aches and, sometimes, nausea, vomiting, and/or diarrhea. It is especially important to test as early as possible once symptoms start, as oral antivirals are only effective if started within five days of symptom onset.
-By EPiC FHI360