Oxygen FAQ
Up to date, expert answers to frequently asked questions (FAQ) about oxygen supply systems, respiratory care and pulse oximetry written by OCC & collaborators.
How to use a pulse oximeter
Ideally, it is best to place the probe on a warm finger on the patient’s non-dominant hand so that the patient can still use their dominant hand without hindrance. For patients with decreased levels of consciousness (e.g. emerging from sedation), the middle finger is a good finger to place the probe because patients are less likely to scratch their face or eyes with this finger. However, if you are unable to get a good reading on this finger, try the other fingers or the other hand until a good waveform is obtained.
References: Lifebox Pulse Oximetry Learning Module
Keywords: finger, placement, location
Different models of pulse oximeters will look slightly different and may have some variation, but many devices will operate in a similar way. The screen of the device will show a reading of the SpO2, the pulse rate, and often the waveform.
- If possible, first remove any nail polish and warm the hands if they are cold.
- Ensure that the probe is connected to the oximeter and that the device is charged.
- Power on the device, and then clip the probe onto the fingertip while keeping the patient’s hand still.
- After a few moments, the screen will display the SpO2.
- If the device shows a pleth, it is important to check that the waveform looks appropriate.
Many pulse oximeters also have buttons which allow the user to control alarm volume, screen display, and turn the device on and off. Now, the patient can continue to be monitored and checked for signs and symptoms of hypoxemia. It is always important to follow the manufacturer’s instructions for use if these are available. Finally, if the device does not seem to be working, try to troubleshoot the issue.
References: Lifebox Pulse Oximetry Learning Module
Keywords: how to use, instructions
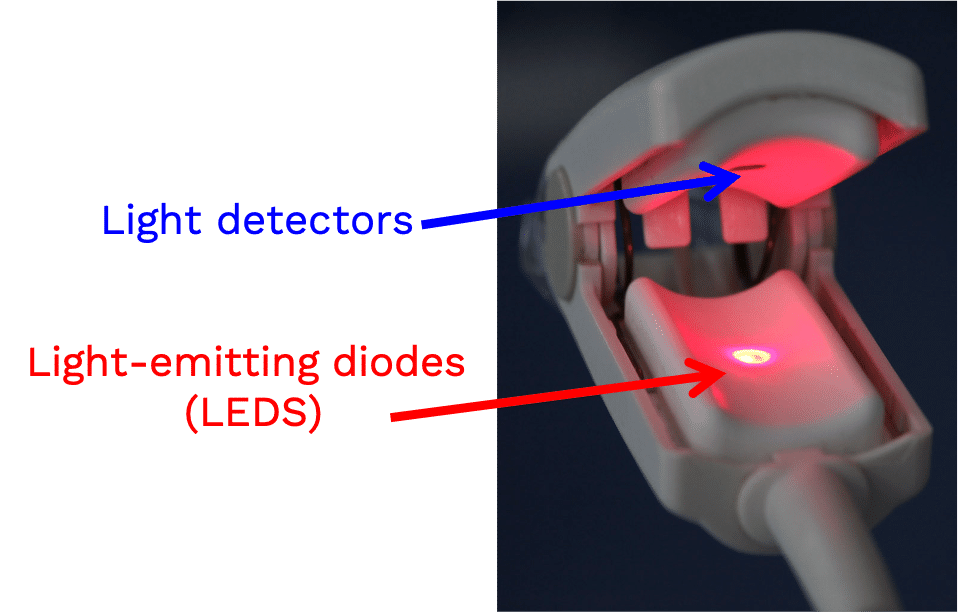
A pulse oximeter is a noninvasive monitoring device that can indirectly measure a person’s functional oxygen saturation (SpO2) and help with early detection of hypoxemia. Pulse oximeters work by either transmitting light through tissue perfused by blood (ideally a site with a dense capillary bed like the fingertip). They are called pulse oximeters because they use the pulsations to discriminate between arterial blood and other tissue or venous blood. The probe on the pulse oximeter has light-emitting diodes (LEDs) which shine at least two types of light, red and infrared. The device is positioned so that the light shines through a translucent part of a patient’s body, such as a fingertip or earlobe, and measures the changing absorbance at each wavelength. Oxygenated hemoglobin absorbs more infrared light, whereas deoxygenated hemoglobin absorbs more red light. Since the absorption of light at these wavelengths differs between oxygenated and deoxygenated blood, the device can determine the absorbances due to just the arterial blood and thereby determine a patient’s peripheral oxygen saturation.
References: Lifebox Pulse Oximetry Learning Module; WHO Using Pulse Oximeters
Keywords: wavelengths, IR, infrared, red, LED
Pulse oximeters can be used to measure many different clinically important values. Some (but not all) pulse oximeters can measure the following:
- Respiratory rate
- Perfusion
- Carboxyhemoglobin
- Methemoglobin
- Hemoglobin concentration
- Pulsatility variation
Keywords: measurement, respiratory rate, pulsatility variation
The optimal target for oxygen saturation (SpO2) in patients with acute hypoxemic respiratory failure is unknown. Hypoxemia causes pulmonary vasoconstriction and pulmonary hypertension in its chronic form, and death when it is acute and severe. Hyperoxemia also causes physiologic disturbances, through toxic reactive oxygen species and absorption atelectasis.1
The World Health Organization (WHO) interim guidance for patients with hypoxemic respiratory failure due to COVID-19 suggests an initial SpO2 target >94% for stabilization, then >90% for non-pregnant patients and 92-95% for pregnant patients, once stable.2 The summary of evidence below suggests another reasonable target might be SpO2 90-96%, and perhaps 92-96% in settings with only intermittent pulse oximetry monitoring, or in patients with darker skin pigmentation.
The evidence
Several recent studies have examined conservative versus liberal oxygenation targets, with mixed results (See Table). In 2016, a before and after stepwise implementation study found a trend toward improved clinical outcomes with SpO2 target of 92-95% and PaO2 target of 55-86 mmHg as compared with earlier higher targets in ICU patients.3 Also in 2016, a single-center, open-label, randomized clinical trial compared targets of SpO2 94-98%/paO2 70-100 mmHg versus SpO2 97-100%/paO2 up to 150 mmHg in ICU patients, and found a mortality benefit with the lower target; however, the study was stopped early due to poor enrollment after a natural disaster, so its results cannot be considered definitive.4
In 2020, two multicenter randomized trials published together in the New England Journal of Medicine produced different results. One compared targets of SpO2 88-92%/paO2 55-70 mmHg with SpO2>=96%/PaO2 90-105 mmHg in ARDS patients, and was stopped early for suggestion of harm in the lower-SpO2 target arm.5 The other compared one arm with a target SpO2<97% with an arm with no maximum target SpO2 limit (lower limit 90% for both arms) in mechanically ventilated patients, and found no difference in ventilator-free days or 180-day mortality.6
Finally, in a 2021 multicenter randomized trial also in the New England Journal of Medicine, investigators randomized almost 3,000 ICU patients with acute hypoxemic respiratory failure to receive PaO2 targets of either 60 mmHg or 90 mmHg.7 The median SpO2 in the higher-target group was 96% (IQR 95-97%), and in the lower-target group was 93% (IQR 92-94%). The study found no difference in 90-day mortality.
The evidence taken together suggests that a target range that avoids both hypoxemia and hyperoxemia may be beneficial. A reasonable example target range based on the above evidence is SpO2 of 90-96%.
Other considerations
Other factors may need to be considered when deciding on a Spo2 target range:
1) Resource-variable settings:
- Lower target saturations can conserve scarce oxygen resources, making more oxygen available for more patients who need it; a hospital Emergency Department in Rwanda found that a target of 90-95% resulted in better oxygen supply reserves for the hospital, as compared with previous higher SpO2 targets.8
- Intermittent pulse oximetry monitoring (versus continuous) could increase the risk for periods of undetected hypoxemia.1 This could be an argument for a slightly higher target, for example 92-96%.
- The accuracy of inexpensive pulse oximeters without regulatory approval is variable.9 Using validated oximeters and following trends in SpO2 rather than individual measurements, and/or checking saturations with more than one device, may help mitigate this concern.
2) Race and skin pigmentation: Data suggest that pulse oximetry more frequently under reports hypoxemia in patients who self-identify as Black, as compared with patients who self-identify as White.10 While more work needs to be done with specific devices and with documentation of a validated range of skin pigmentations, it may be reasonable currently to target higher ranges in patients with darker skin tones, for instance 93-96%.
3) Altitude: Geographic variation in elevation above sea level may necessitate adjustment in SpO2 targets. Baseline SpO2 values in healthy people will be lower at higher elevation due to decreases in the partial pressure of oxygen.
4) Other conditions: The recommendations here are related to acute hypoxemic respiratory failure. Other conditions may necessitate higher or lower targets. For example, a COPD exacerbation in a patient with chronic hypercarbic respiratory failure should likely have a lower target of 88% to avoid worsening of hypercarbia. Pregnant patients generally have higher SpO2 goals.2
British Medical Journal (BMJ) Rapid Recommendations, 2018
Acutely Ill Medical Patients
- Target SpO2 < 96% for acutely ill patients requiring supplemental oxygen
- Do not start supplemental oxygen in patients with SpO2 93-100%
American Association of Respiratory Care, 2002
All patients in acute care facility
- Provide supplemental oxygen for SpO2 < 90%
British Thoracic Society, 2017
Acute medical conditions Provide oxygen if SaO2 <94% for most acutely ill patients; <88% for patients with hypercapnia 98% for most patients, 92% for patients with hypercapnia
- Provide oxygen if SaO2 <94% for most acutely ill patients; <88% for patients with hypercapnia
- Upper limit of target range 98% for most patients, 92% for patients with hypercapnia
Thoracic Society of Australia and New Zealand, 2015
Acute medical conditions
- Provide oxygen if SpO2 <92% for most acutely ill patients; <88% for patients with COPD and some other forms of chronic respiratory failure
- Target SpO2 92-96%
World Health Organization, 2020[ER3]
Patients with Severe Acute Respiratory Infection (SARI)
- Provide oxygen if SpO2 <90%, no upper limit specified
- Target SpO2 88-93% in patients with ARDS from SARI
National Institute of Health, 2020
Patients with COVID-19 infection
- Target SpO2 92-96%
Society of Critical Care Medicine
Patients with COVID-19 Infection
- Suggest starting supplemental oxygen if SpO2 <92 and recommend starting supplemental oxygen if SpO2 <90
- Target SpO2 92-96%
- Strongly recommend upper limit of 96%
ARDSNet, 2008
Intubated patients with ARDS
- 88-95%
Citations
- Angus DC. Oxygen Therapy for the Critically Ill. N Engl J Med 2020;382:1054-6.
- World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: Interim guidance. 2020 March 13.
- Helmerhorst HJ, Schultz MJ, van der Voort PH, Bosman RJ, Juffermans NP, de Wilde RB, van den Akker-van Marle ME, van Bodegom-Vos L, de Vries M, Eslami S, de Keizer NF, Abu-Hanna A, van Westerloo DJ, de Jonge E. Effectiveness and Clinical Outcomes of a Two-Step Implementation of Conservative Oxygenation Targets in Critically Ill Patients: A Before and After Trial. Crit Care Med 2016;44:554-63.
- Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Morelli A, Antonelli M, Singer M. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. Jama 2016;316:1583-9.
- Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, Quenot JP, Pili-Floury S, Bouhemad B, Louis G, Souweine B, Collange O, Pottecher J, Levy B, Puyraveau M, Vettoretti L, Constantin JM, Capellier G. Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med 2020;382:999-1008.
- Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, Finfer S, Freebairn R, King V, Linke N, Litton E, McArthur C, McGuinness S, Panwar R, Young P. Conservative Oxygen Therapy during Mechanical Ventilation in the ICU. N Engl J Med 2020;382:989-98.
- Schjørring OL, Klitgaard TL, Perner A, Wetterslev J, Lange T, Siegemund M, Bäcklund M, Keus F, Laake JH, Morgan M, Thormar KM, Rosborg SA, Bisgaard J, Erntgaard AES, Lynnerup AH, Pedersen RL, Crescioli E, Gielstrup TC, Behzadi MT, Poulsen LM, Estrup S, Laigaard JP, Andersen C, Mortensen CB, Brand BA, White J, Jarnvig IL, Møller MH, Quist L, Bestle MH, Schønemann-Lund M, Kamper MK, Hindborg M, Hollinger A, Gebhard CE, Zellweger N, Meyhoff CS, Hjort M, Bech LK, Grøfte T, Bundgaard H, Østergaard LHM, Thyø MA, Hildebrandt T, Uslu B, Sølling CG, Møller-Nielsen N, Brøchner AC, Borup M, Okkonen M, Dieperink W, Pedersen UG, Andreasen AS, Buus L, Aslam TN, Winding RR, Schefold JC, Thorup SB, Iversen SA, Engstrøm J, Kjær MN, Rasmussen BS. Lower or Higher Oxygenation Targets for Acute Hypoxemic Respiratory Failure. N Engl J Med 2021.
- Sutherland T, Moriau V, Niyonzima JM, Mueller A, Kabeja L, Twagirumugabe T, Rosenberg N, Umuhire OF, Talmor DS, Riviello ED. The “Just Right” Amount of Oxygen. Improving Oxygen Use in a Rwandan Emergency Department. Ann Am Thorac Soc 2019;16:1138-42.
- Lipnick MS, Feiner JR, Au P, Bernstein M, Bickler PE. The Accuracy of 6 Inexpensive Pulse Oximeters Not Cleared by the Food and Drug Administration: The Possible Global Public Health Implications. Anesth Analg 2016;123:338-45.
- Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial Bias in Pulse Oximetry Measurement. N Engl J Med 2020;383:2477-8.
Pulse oximetry plays a key role in the diagnosis, triage and management of COVID-19 patients. This includes when to initiate hospitalization or oxygen therapy and when to escalate or deescalate oxygen therapy. This can be an essential tool for conserving oxygen supply as well.
Hypoxemia is a key finding in many patients with COVID-19. Up to 80% of patients with COVID-19 may have only mild symptoms or be completely asymptomatic. Common initial symptoms of COVID-19 infection include fever, cough, headache, fatigue, and myalgias. About 15% of infected patients develop severe disease, and about 5% develop critical illness – these patients may require supplemental oxygen. It has been reported that many patients with COVID-19 have more significant hypoxemia than their symptoms might suggest, which has been referred to as “happy” or “silent” hypoxia. Therefore, it is essential to check SpO2 for all patients with known or suspected COVID-19 infection who are symptomatic or report for medical evaluation.
References: Lifebox Pulse Oximetry Learning Module
Keywords: COVID-19, symptomatic, shortness of breath, SOB, coronavirus, oxygen
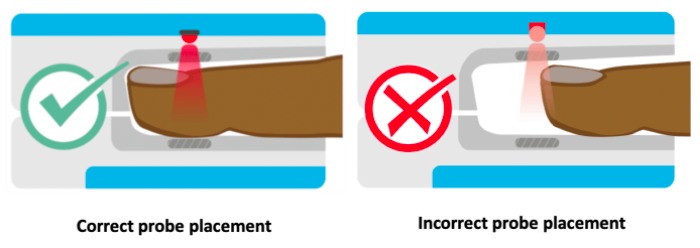
The locations for placing pulse oximeters vary by the type of device and probes available, including whether it is a transmittance or a reflectance device. Transmittance devices shine light through a part of the body, so they must be placed on a relatively thin or translucent area such as a fingertip, earlobe, nose or the foot of an infant. It is important that the body part is placed far enough into the probe so that the light shines through the tissue rather than to the side of it. Reflectance devices do not require light to pass through tissue, so they can be placed in a variety of locations including the patient’s forehead, wrist, foot, or chest.
References: Lifebox Pulse Oximetry Learning Module
Keywords: body part, location, placement, finger